Drugmaker Moderna asked the Food and Drug Administration on Thursday to authorize a fourth shot of its COVID-19 vaccine as a booster dose for all adults.
The request is broader than rival pharmaceutical company Pfizer’s request earlier this week for the regulator to approve a booster shot for all seniors.
In a press release, the company said its request for approval for all adults was made “to provide flexibility” to the Centers for Disease Control and Prevention and medical providers to determine the “appropriate use” of a second booster dose of the mRNA vaccine, “including for those at higher risk of COVID-19 due to age or comorbidities.”






 Primary Election Day
Primary Election Day
 Severe Weather Threat for Lakes Region Increases for Wednesday, Friday
Severe Weather Threat for Lakes Region Increases for Wednesday, Friday
 Harrison Man Charged After Pursuit in Baxter County
Harrison Man Charged After Pursuit in Baxter County
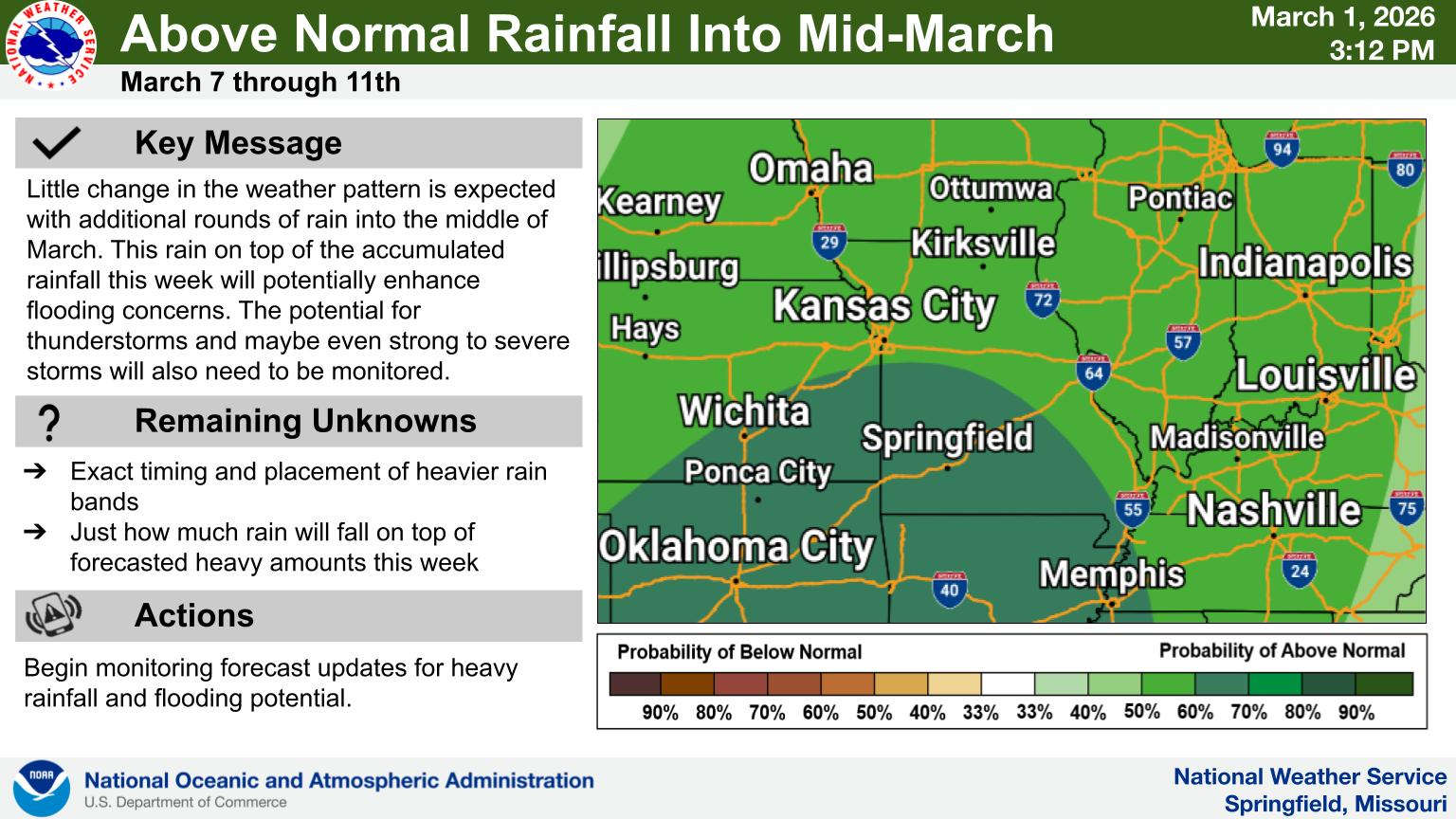 Rainy Pattern in Place, Severe Storms Possible Later This Week
Rainy Pattern in Place, Severe Storms Possible Later This Week
 Early Voting Last Day, Primary Election Tuesday
Early Voting Last Day, Primary Election Tuesday